“Cloning Said to Yield Human Embryos”
This New York Times article by Andrew Pollack from January 18th, 2008 reports on a small, San Diego-based biotech company called Stemagen which was the first to create human embryos from the skin cells of two adult males. Some experts quoted in the article were skeptical about the significance of the findings since the embryos only grew to the blastocyst stage and it’s unclear whether (a) they were sufficiently healthy to survive had they been implanted in a womb and (b) embryonic stem cells could actually be derived from the clones. Nonetheless, the chief executive of Stemagen says that the company has “at least shown the opening to the cave that has the holy grail” (the ‘holy grail’ being the ability to create, via cloning, patient-specific embryonic stem cells
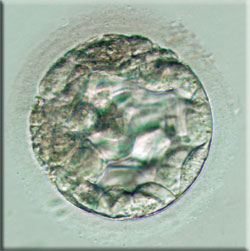 in order to treat diseases such as Parkinson’s and diabetes).
in order to treat diseases such as Parkinson’s and diabetes).As previously discussed (see post: “Some Facts About Cloning”), the goal of this kind of cloning, referred to as therapeutic cloning, is not to clone human beings, but to create embryos in order to harvest stem cells that can be used to treat diseases. Stem cells have the potential to differentiate into any type of cell in the body, which is why they have great promise in treating diseases such as Alzheimer’s. The NYT article does say that the official statement from Stemagen is that the company is “not interested in creating cloned babies, something that is illegal in places and morally repugnant to many people.” However, in my opinion, the article does not draw a clear enough line between therapeutic and reproductive cloning. Or rather, for the average reader with no knowledge of different kinds of cloning, it muddies the line with sentences like “Although the embryos grew only to a very early stage, the work could also theoretically be seen as a step toward creating babies that are genetic copies of other people.” This ambiguous kind of wording could influence public perception of therapeutic cloning and link it, to its detriment, with reproductive cloning. Since the majority (90% as reported in the TIME poll cited in post: “Cloning for the Commoner”) of Americans think that cloning humans is a bad idea, tying therapeutic cloning with the cloning of people can only do harm to public perception of therapeutic cloning technologies.
One aspect of this article I particularly liked was that it not only presented Stemagen’s claims, but also presented the limitations of the study. So the overall message isn’t that science has made a huge leap in cloning technology, but rather, a small step has been made in the right direction (or as Stemagen puts it, we’ve been “shown the opening to the cave”). Maybe not as concrete of an advance as one would like, but I think in the end, more representative of how science really works. The article offers feedback on the researchers’ claims from different experts, some who are extremely optimistic about the results, and some decidedly less so. This process of peer review is essential to science in general, but particularly in the realm of public health debates. When policymakers need to decide whether a particular technology is worth investing money in, or indeed, as is the case with cloning, whether the technology is morally right, they should be informed of the context of each finding. In order for the public and the policymakers to make good decisions about the development and use of cloning technology, such as in the case of the new "Human Cloning Prohibition Act of 2009" (see below), they need to be informed by experts of what each new finding really means: good and bad.
A. Suchecka

If an organ is needed and therepeutic cloning is the way the patient wants to go, where is the organ grown since the embryo is not implanted into a woman's womb? Is it grown in a chemical or petri dish?
ReplyDelete